|
Things you should do
|
Remind any doctor, dentist, pharmacist or nurse you visit that you are using NIVESTIM.
Keep all your doctor’s appointments so that your health can be monitored.
Call your doctor straight away if you become pregnant.
Go straight to the hospital if you notice any signs or symptoms of infection.
|
|---|---|
|
Things you should not do
|
Do not stop using this medicine or lower the dosage without checking with your doctor.
Do not use NIVESTIM to treat any other complaint unless your doctor tells you to.
|
|
Driving or using machines
|
Be careful driving or using any machines or tools until you know how NIVESTIM affects
you.
|
|
Looking after your medicine
|
Keep NIVESTIM in a refrigerator at a temperature of 2°C to 8°C.
Keep your medicine in its pack until it is time to use it. Protect it from light.
|
Do not use NIVESTIM:
Check with your doctor if you:
Pregnancy and breastfeeding
Use in Children
How much NIVESTIM to use
When to use NIVESTIM
How to use NIVESTIM
Equipment required for administration
Where to inject

Things to do before you inject
1. Find a clean, flat working surface, such as a table, where you can inject undisturbed.
2. Remove the carton containing the NIVESTIM pre-filled syringes from the refrigerator.
3. Remove the blister tray containing the pre-filled syringe from the carton. When the carton contains blister trays with more than one pre-filled syringe, tear off the blister tray containing one pre-filled syringe along the perforated part, and return the rest of the blister trays containing pre-filled syringes to the carton and return the carton to the refrigerator.
4. Open the blister tray containing the pre-filled syringe by peeling away the lid from the blister tray. Remove the pre-filled syringe from the blister tray by grasping from the syringe body.
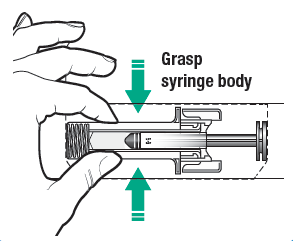
5. Check the syringe to make sure that the needle guard is covering the barrel of the pre-filled syringe. Do not push the needle guard over the needle cover before the injection. This may activate or lock the needle guard. If the needle guard is covering the needle that means it has been activated.
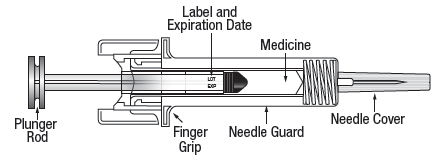
6. Check that the solution is clear, colourless and practically free from visible particles. Do not inspect the product through the plastic of the safety device.
7. Check the date on the syringe label to make sure that the medicine has not passed the expiry date.
8. For a more comfortable injection allow the pre-filled syringe to reach room temperature (approximately 25°C). This will take 15-30 minutes.
b. Do not shake the syringe.
c. Do not remove the needle cover until you are ready to inject.
9. Make sure you have your puncture-resistant sharps container nearby.
10. Wash and dry your hands thoroughly.
Do not use the NIVESTIM syringe if:
How to prepare your injection – NIVESTIM Ready to Use Syringe
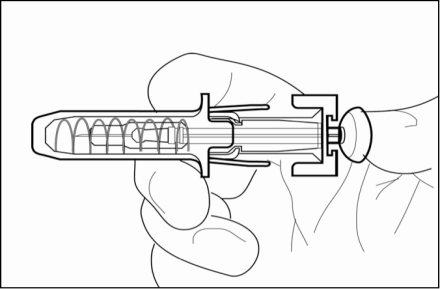
1. Hold the pre-filled syringe by the body of the needle guard with the needle cover pointing up – this helps reduce the amount of medicine that may leak out of the needle.
b. Do not pull back on the plunger at any time.
c. Do not remove the needle cover from the pre-filled syringe until you are ready to inject your medicine.
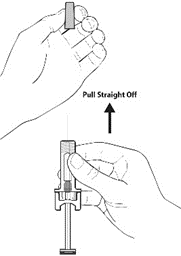
2. Carefully remove the needle cover by holding the barrel and pulling the cover straight off and away from your body carefully without twisting it. Throw away the cover. Do not recap the needle. Do not push the plunger or touch the exposed needle or shake the syringe.
3. Check the dose (in mL) that your doctor has prescribed and locate the correct volume mark on the syringe barrel. Carefully push the plunger until the grey upper edge of the plunger reaches the correct volume mark. This will push the air and any excess liquid out of the syringe.
4. Double-check that you have the correct dose.
1. Clean the site where the injection is to be made with an alcohol swab, moving the alcohol swab in an expanding circle and allow the site to dry.

2. Pinch a large area of skin between your thumb and forefinger, to create a firm injection site.
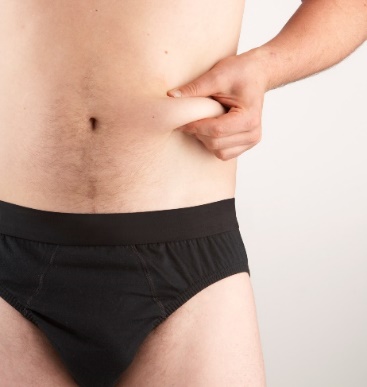
3. With your other hand, pick up the pre-filled syringe and hold it as you would a pencil.
4. Use a quick "dart-like" motion to insert the needle directly into the skin (at an angle of 45° or as advised by your doctor, nurse or pharmacist).
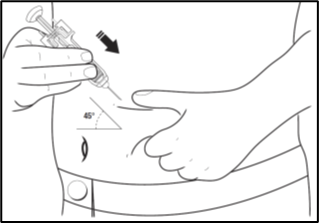
5. After the needle is in, pull back the plunger very slightly. If blood comes into the syringe, the needle has entered a blood vessel. Remove the needle.
6. Select another site, clean the new site with an alcohol swab and reinsert the needle. Again, pull back the plunger very slightly to check for blood. If blood does not appear in the syringe, you are ready to inject.
7. Gently push down the plunger until all the contents of the pre-filled syringe have been emptied.
8. Withdraw the needle and using the alcohol swab apply pressure for several minutes to the injection site.
9. Do not put the needle cover back on the used syringe. You cannot reuse the syringe.
10. Ensure needle guard covers the needle according to instructions for Active Needle Guard or Passive Needle Guard (below).
11. Discard the used syringe into an approved, puncture-resistant, sharps container.
Use of Active Ultrasafe Needle Guard for NIVESTIM 120 µg/0.2mL solution for injection
1. Perform the injection using the technique described above.
2. When you have completed the injection, slide the needle guard forward until the needle is completely covered (device ‘clicks’ into place).
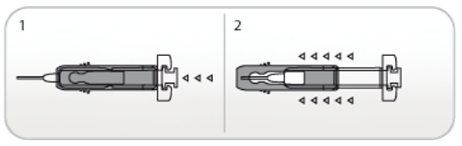
Use of Ultrasafe Passive Needle Guard for NIVESTIM 300 µg/0.5mL solution for injection and NIVESTIM 480 µg/0.5mL solution for injection
1. Perform the injection using the technique described above.
2. Depress the plunger while grasping the finger flange until the entire dose has been given. The passive needle guard will NOT activate unless the ENTIRE dose has been given.
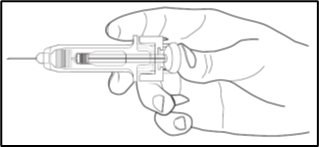
3. Remove needle from your skin, then let go of the plunger and allow syringe to move up until the entire needle is guarded and locks into place.
How long to use NIVESTIM for
If you forget to use NIVESTIM
If you use too much NIVESTIM
(by calling 13 11 26), or
Things you should do
Go straight to the hospital if you:
There are many ways an infection may show itself. You should watch for:
Things you should not do
Driving or using machines
Looking after your medicine
When to discard your medicine
Getting rid of any unwanted medicine
Side effects
|
Side effects
|
What to do
|
|---|---|
|
Some of these are known side effects of chemotherapy and may not be related to NIVESTIM:
Muscle and Skeleton:
temporary bone pain, such as in the lower back or in the long bones of the arms or
legs.
This pain is usually relieved with non-prescription painkillers, like paracetamol.
If you continue to have bone pain even after having taken this form of pain relief,
you should speak to your doctor, as you may need a prescription medication.
back pain
pain, swelling, warmth or stiffness of joints
worsening of existing arthritis
muscle pain
muscle spasms
Gut and Digestion:
abdominal discomfort
diarrhoea or constipation
nausea (feeling sick) and/or vomiting
Blood:
pink, red or blue/purple spots or bumps on the skin
bleeding or bruising more than usual, severe nose bleeds
reddish or purplish blotches under the skin
Eyes, ears and mouth:
sore throat
sore mouth, mouth ulcers
Nervous System:
numbness
tingling in the hands and feet
Skin:
redness, swelling or itching at the site of injection
skin disorders - worsening of existing symptoms
skin rash or red, itchy or sore spots
swelling of hands, legs, ankles, feet or any other part of the body
General:
cough, breathlessness
hair loss
headache
tiredness, looking pale
loss of appetite
unusual weakness
difficulty sleeping
|
Speak to your doctor if you have any of these side effects and they worry you.
|
Serious side effects
|
Serious side effects
|
What to do
|
|---|---|
|
General:
chest pain
fever
general feeling of tiredness
easy bruising or bleeding
left shoulder tip pain
frequent infections
Dizziness or feeling light-headed
Faintness
Lungs and upper airways:
coughing up blood or mucus, bleeding from the lung
breathing problems such as shortness of breath, rapid breathing
Gut, Digestion and Urine-related:
pain in the upper left side of the stomach (abdomen)
swelling of your stomach-area (abdomen) and feeling of fullness
less frequent urination
blood in the urine
Skin:
fever and painful skin lesions, most commonly on your arms, legs and sometimes on
your face and neck
swelling or puffiness
Heart-related:
fever, chest or abdominal pain, malaise and back pain. These could be symptoms of
inflammation of your aorta (the large vessel that transports blood from your heart
to your body). Tell your doctor if you experience these symptoms.
Symptoms of severe allergic reaction:
pinkish, itchy swellings on the skin, also called hives or nettle rash
swelling of the face, lips, mouth or throat which may cause difficulty in swallowing
or breathing
shortness of breath, wheezing
light-headedness, dizziness or fainting
rapid pulse, sweating
|
Call your doctor straight away, or go straight to the Emergency Department at your
nearest hospital if you notice any of these serious side effects.
|
Reporting side effects
What NIVESTIM contains
|
Active ingredient
(main ingredient)
|
filgrastim (rbe)
|
|---|---|
|
Other ingredients
(inactive ingredients)
|
glacial acetic acid
polysorbate 80
sodium hydroxide
sorbitol
water for injections |